King Troubleshooter – Miniprep edition
King Troubleshooter – Miniprep edition
One time during lab meeting toward the end of my graduate training, Alan (my PhD advisor) was telling the group that unless they were me (since apparently he noticed that I was good at solving my own problems), that they should be going to him for help troubleshooting experiments. This praise was noteworthy to people in the lab (since such praise wasn’t common), and it became a little bit of a joke afterwards that I was “King Troubleshooter”.
Well, fast forward 4 years and I’m no longer a senior grad student but a senior postdoc. Experiments feel like they’re all going pretty well, but suddenly all of my sequencing reactions started failing. Nanodropping revealed that my recent batches of Qiagen minipreps were all giving me little if any DNA (~10-30 ng/ul, with pretty poor 260/280 ratios). Definitely very unsettling, and really frustrating when an usually automatic component of your cloning pipeline inexplicably breaks down.
I complained to Melissa, one of the grad students in the lab, which gave me an opportunity to summarize and describe what I knew: I was definitely seeing alkaline lysis (lyseblue changing blue, and solution becoming viscous), which was seemingly neutralized with the neutralization buffer (viscous solution turns into solution with white flocculent precipitate that could be pelleted). Thus, 1) either my antibiotic went bad (so little plasmid DNA in bacterial culture), 2) plasmid DNA wasn’t binding the column, or 3) plasmid DNA was being premature eluted (such as during the wash step). Jokingly, knowing that an undergrad had recently gotten good sequencing (and thus good minipreps) using a different Thermo miniprep kit we have in the lab, I said that I should just combinatorially mix and match all the reagents to figure it out what was failing. I chuckled, walked away, and realized… wait. I totally could do this experiment in a reasonable number of samples and actually troubleshoot the problem.
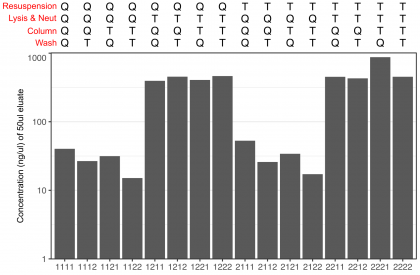
It took me less than 30 min to get my answer. To guard against issue 1 from above (bad antibiotic) giving me all bad minipreps (which I suppose could have been informative as an outcome, albeit kind of boring), I instead found a frozen midiprep cuture I no longer needed (but knew had given me plenty of plasmid when prepped before), which I used that as my starting culture. To keep the number of combinations to a reasonable 16 (rather than 32), I linked the lysis and neutralization buffers (so both Qiagen, or both Thermo). “Q” stands for the Qiagen kit (the one that was failing), and “T” for the Thermo kit (which I knew seemed to work) in the below plot.
So the pattern was ultra-clear: Combinations that had used the Qiagen lysis and neutralization buffers didn’t work, while those that used the Thermo buffers worked perfectly well (giving me a good 10ug per prep). As an added benefit, this experiment showed that not only were both kits pretty comparable in yield (when working well), that all of the components were indeed essentially completely interchangeable.
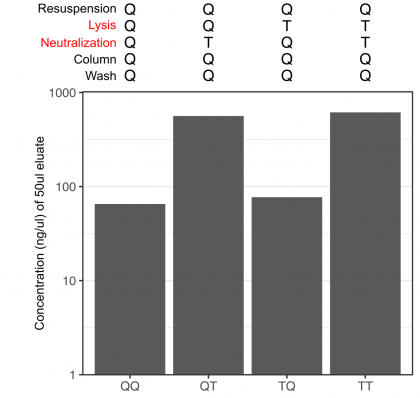
Well, now knowing this, I decided to start minipreps for actual plasmids I was cloning using one of the combinations that worked fine, when I realized I could add 4 additional samples to my set to further understand the situation.
OK, so this showed that only the neutralization buffer (and not the lysis buffer) was causing minipreps to fail.
So the story essentially ends here. I pH’d the neutralization buffers, and they were 4.3 and 5.5, which is roughly as expected / formulated. I’m guessing some other component of the buffer must be preventing the plasmid DNA from being captured on the column; perhaps by not letting the plasmid DNA from rehybridizing for binding to the column.
Overall, I thought this was a success. I was able to take a frustrating situation, and instead of giving up or completely avoiding the problem, I troubleshot it far enough to isolate the problematic component, while getting some information about how comparable or interchangeable these kits were along the way. While I still don’t know why that specific bottle of neutralization buffer no longer works, I now know this is a potential mode of failure in alkaline lysis, which I certainly would not have guessed beforehand (my hypothesis going into this experiment was that the wash buffer had somehow caused premature elution). Lastly, it gives me confidence that however big or small the problem I run into, that I can take a reasonable course of steps to successfully troubleshoot it.
Some final notes:
Thanks to Melissa (and Kate) who were still in the lab late on a Friday afternoon, giving me an audience for thinking through this problem aloud.
In no way do I think this is a general Qiagen problem. Overall, I really like Qiagen kits. For whatever reason, this specific bottle on my bench stopped working.