Lab Hack: SYBR Safe Excitation with Blue LEDs
Bottom Line (Up Front): Blue LEDs alone, while they emit more background signal, can strongly excite SYBR Safe DNA dyes, and can be implemented at a fraction of up-front cost (<$20) than purchased equipment. While hardly replacing purchased equipment, a hand-held setup can be made for quickly illuminating and quality-checking agarose DNA gels.
I run a lot of DNA gels, and I’ve found SYBR Safe to be quite useful. While still a DNA binding dye, the chemical itself is supposed to be many times safer than Ethidium Bromide, and its use is clearly safer since you don’t have to expose yourself to UV-light when gel purifying DNA bands. Instead, SYBR Safe is excitated by blue light.
Like most lab equipment, SYBR Safe illuminating devices are rather expensive by everyday standards: the “name brand” Safe Imager™ 2.0 Blue-Light Transilluminator from ThermoFisher is $1450, while the “off brand” Dark Reader Transilluminators from Clare Chemical Research (what our lab uses) range from $475 to $2128.
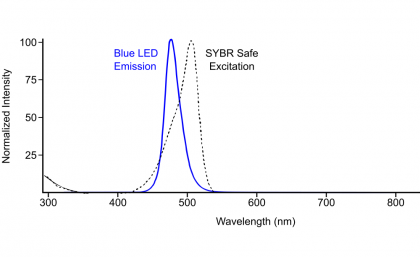
Since it’s just supposed to be blue light, I wondered if I could hack something together for much cheaper. I looked to see what the emission spectrum of a blue LED was, and found it to be pretty narrow and overlay quite well with the SYBR Safe excitation spectrum. (See the figure I made, below):
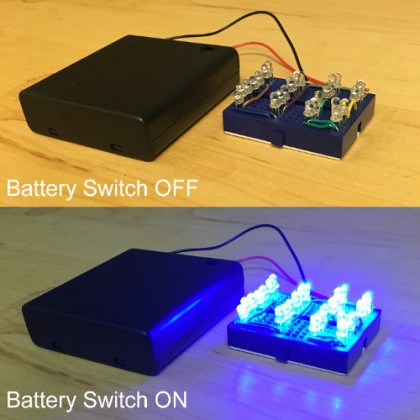
Thus, I bought a pack of 50 Blue LEDs for $5, a pack of two 6V AA battery cases for $3, and pack of 6 mini breadboards ($6) for hooking everything together (with some simple electronics wire I had laying around). I hooked up the LEDs as two sets of 13 parallel LEDs, wired in series (so a super simple layout, with no resistors or anything). What I whipped together quickly for about $5 in actual parts off of Amazon ended up looking like this:
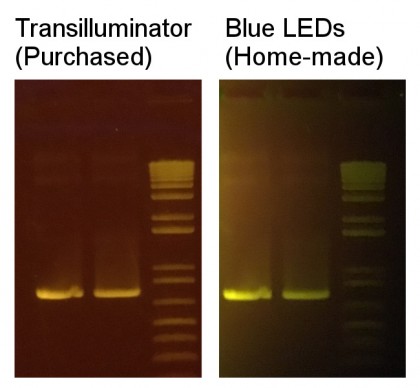
I was already running an agarose gel looking at an amplified PCR product, so I looked at how the SYBR Safe – stained bands looked either excited by the purchased “Dark Reader” Transilluminator, or the LED setup I had rigged together. I used the amber filter that came with the “Dark Reader” in either case, and the results looked like this (captured on my iPhone):
Overall, the amount of signal I could achieve appeared relatively comparable. As a caveat though, the blue LEDs still did emit a fair amount of light that made it past the amber filter, so I couldn’t hold it below the gel and “transilluminate” like the “Dark Reader” (I would see the LEDs shining, obfuscating the DNA bands). On the other hand, if I held it over the gel down at an angle, I still got decent chemical excitation without flooding my eyesight with “background” light. Furthermore, my setup resulted in somewhat localized excitation; this is the reason that the ladder, which was aimed at rather indirectly, was fainter in the image above. It’s quite possible that adding a blue transmitting filter after the LEDs would further narrow the wavelengths and get rid of the remaining background problem (such as bleed-through of greenish emitted light), but I didn’t take this part of the project any further.
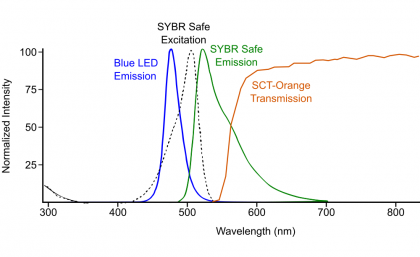
I did still wonder whether I could turn it into a totally home-made setup, and replace the amber filter needed to most clearly observe the SYBR Stain dye. The ThermoFisher SafeImager Glasses are $52, and the Clare Chemical Research Dark Reader Glasses are $88. I tried a couple of different items I found on Amazon. I first bought these MODA Blue-Blocking Sunglasses for $7. They worked, but the “sunglasses” aspect blocked too much desired light to see the DNA bands really easily. I also tried these UVEX SCT-Orange glasses for $7.50. Conveniently, these glasses came with a transmission/filtering spectrum from the company, which I’ve overlaid onto my previous figure, which shows the general principle of the wavelengths I’m trying to block / retain:
These also worked, and didn’t diminish overall signal like the MODA sunglasses did (when looking at actual DNA bands), but they had a different undesirable feature of really lighting up the plastic on the glasses such that it raised the overall background of my eyesight. With the UVEX glasses, if I held the LED lights just right, I could see decent illumination and resolution of stained DNA bands, but it was so much of a hassle I didn’t really count it as any kind of practical success. I gave up here, so I never quite found any good cheap replacement for the amber filter provided by the biotech companies… and I ended up with a couple pairs of blue-blocking glasses I could wear for looking at my computer / phone screen before bed.
In conclusion, while not all that practical, I think I still did succeed in recreating the SYBR Safe illumination package in principle. Nothing I did here TRULY replaced the products provided by the biotech companies, so people should definitely buy these equipment packages if they want to go down this DNA staining route. While the glasses I tested didn’t work all that well, the LEDs worked reasonably well for the little money and effort I put into it. Thus, I would actually consider using something like the LED setup I made here (with the purchased amber filter) just to quickly look at how far my gel had run, before taking it down the hall to the geldoc to take a real picture.
Thanks for sharing. We used a blue acrylic underneath and worked very very well. Details here: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0187163
Saludos